Animation Cathode Ray Experiment

History Of Atoms Storyboard By 52a16d5b

Click Here If Animations Are Not Working Ppt Download
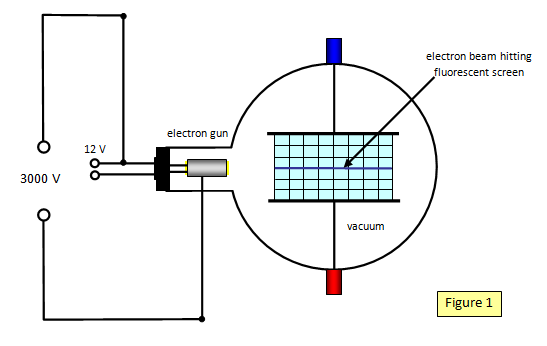
Schoolphysics Welcome
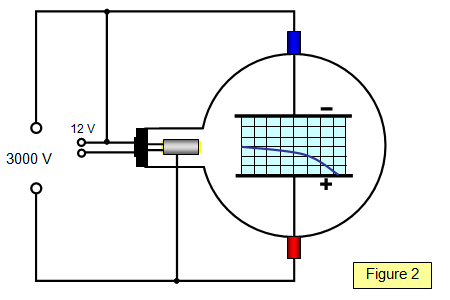
Schoolphysics Welcome
Chemistry Technology Storybvoard Storyboard By Kailahg

The Atomic Theory Of Matter Summary
→This experiment helped in the discovery of the proton.

Animation cathode ray experiment. Rutherford gold foil experiment. (Right-click here to download). Thomson (1856 – 1940) J.
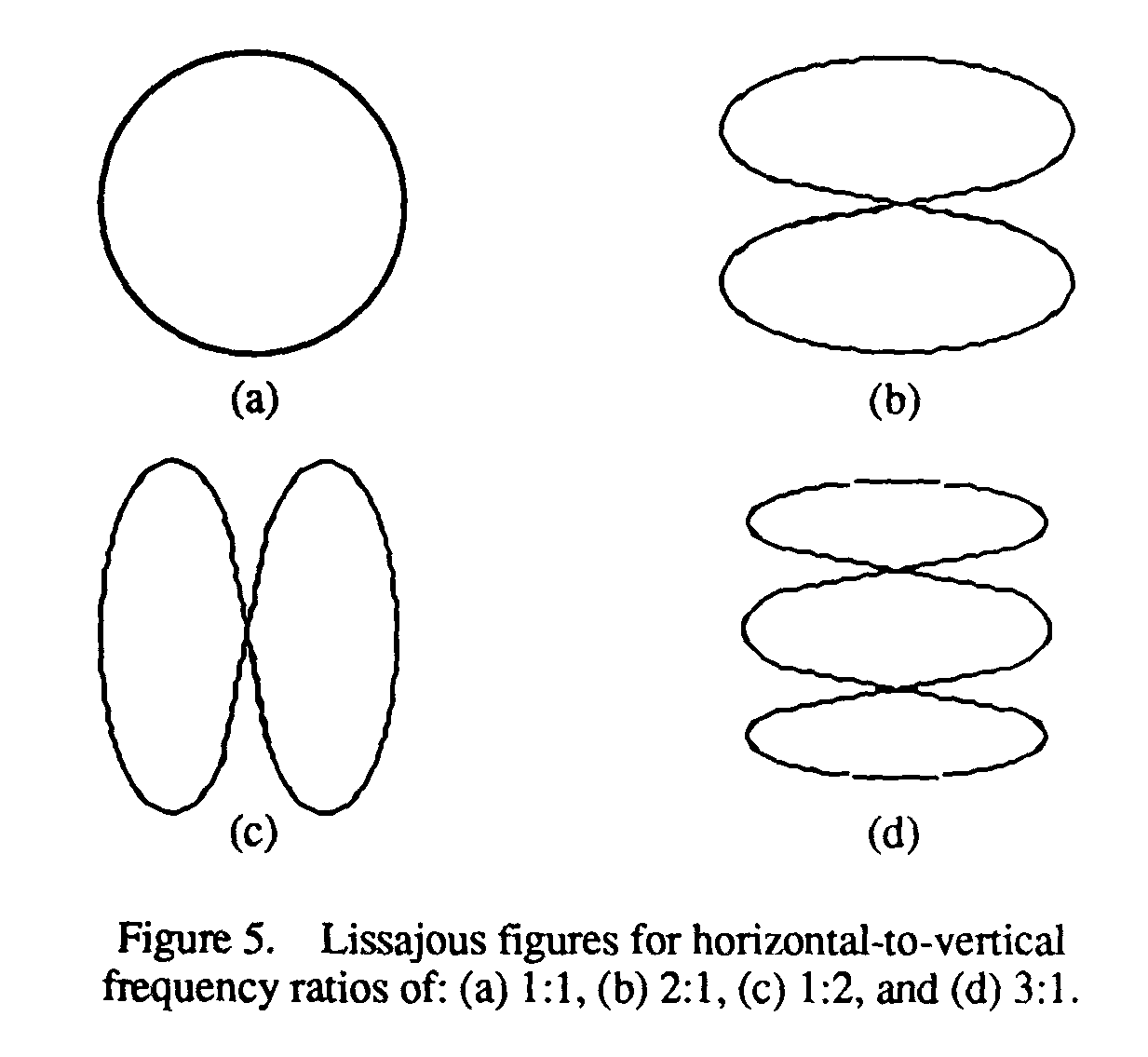
The waveform is generated in such a way that the amplitude of the signal is represented along Y-axis and the variation in the time is represented along X-axis. In 17, British physicist J. What is the principle of operation of cathode ray oscilloscope?.
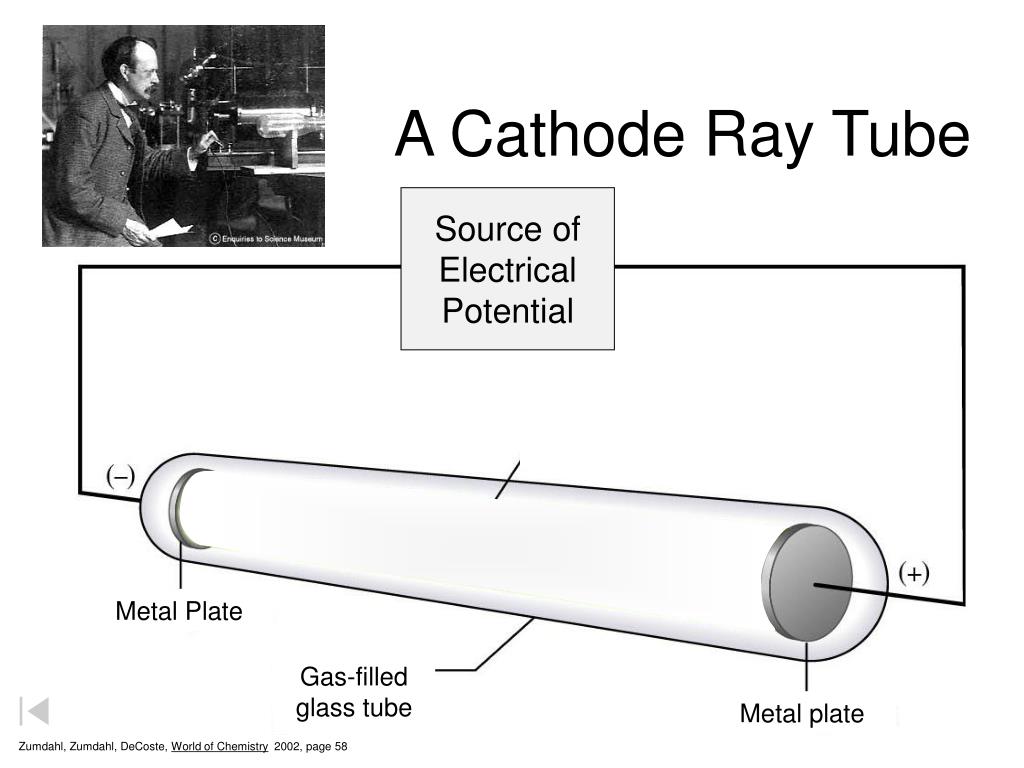
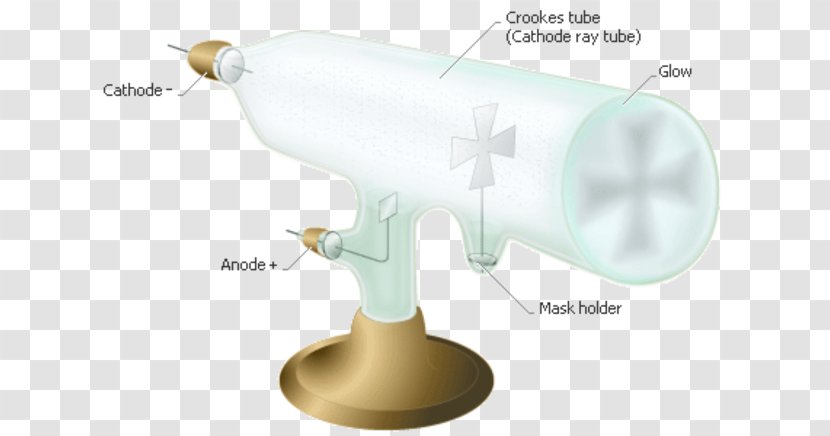
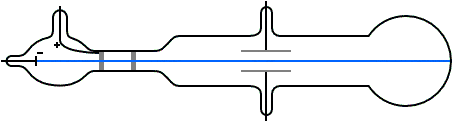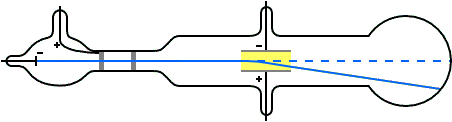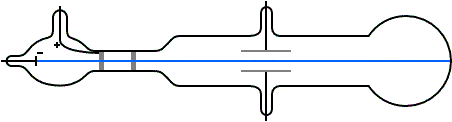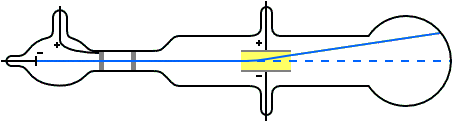
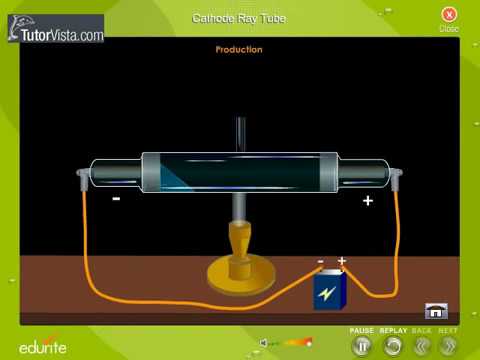
It is a vacuum sealed tube with a cathode and anode on one side. There is a side tube connected to a vacuum pump to reduce pressure. The picture below illustrates the operation of a Crookes tube in a schematic way.
Chapter 3 - Stoichiometry. It is a glass tube from whichmost. They were first observed in 1869 by German physicist Julius Plücker and Johann Wilhelm Hittorf, and were named in 1876 by Eugen Goldstein Kathodenstrahlen, or cathode rays.
Click on the portion of the diagram where the television screen would be located. BUT the maltose cross provided a distinct well formed shadow on the end of the tube. Thomson’s Cathode Ray Experiment.
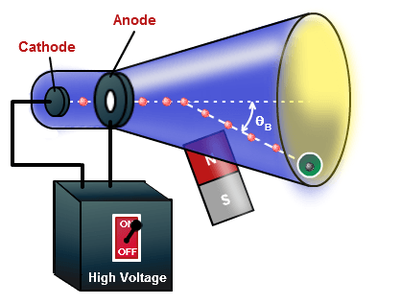
In 1878 William Crooks observed that a beam of light travels from cathode to the anode, when he lowered the pressure of the Crooks Tube with a gas to 1Pa. Your job is to measure the deflection of the beam under different conditions and then determine the ratio of the charge of the particles in the beam to the mass of the particles in the beam. Chapter 9 - Models of Chemical Bonding Rutherford's Experiment 2 Ionic Versus Covalent Bonds:.
Discovery of the electron and nucleus. The term Crookes tube is also used for the first generation, cold cathode X-ray tubes, which evolved from the experimental Crookes tubes and were used until about 19. A class activity sheet accompanies the cathode ray tube demonstrations and experiments.
Thomson, is one of the most well-known physical experiments that led to electron discovery. If you then want to know either the charge or the mass of an electron, you need to have a way of measuring one or the other independently. You can create videos from my animations and place them, for example on youtube.
By Staff Writer Last Updated Apr 10, 3:47:37 AM ET. Flash Animations for Physics > Thomson's experiment. $\endgroup$ – matt_black Dec 13 '18 at 14:05.
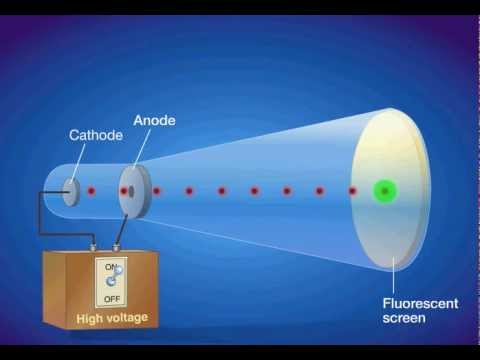
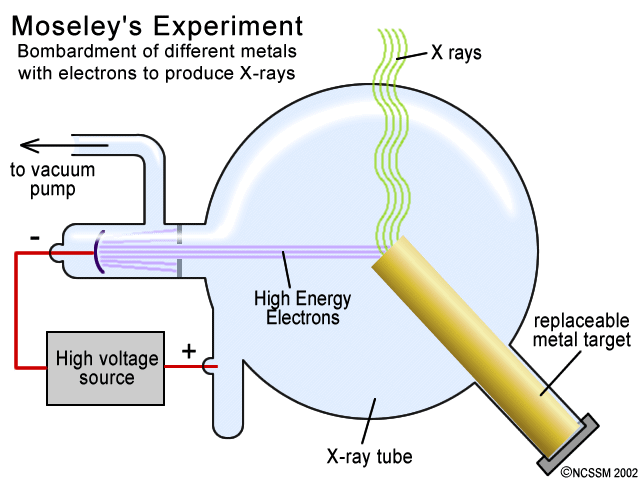
Thompson, conducted the cathode ray tube experiment to prove that rays emitted from an electron gun are inseparable from the latent charge. The apparatus of his experiment is called the cathode-ray tube (CRT). Cathode rays focused on a hard target (anticathode) produce X-rays or focused on a small object in a vacuum generate very high temperatures (cathode-ray furnace).
Cathode Ray Tube Emission Spectrum JJ Thomson's Experiment:. In fact, if you are reading this on a non-flat panel desktop computer monitor, or have ever viewed a non-flat screen television. A portrait of J.
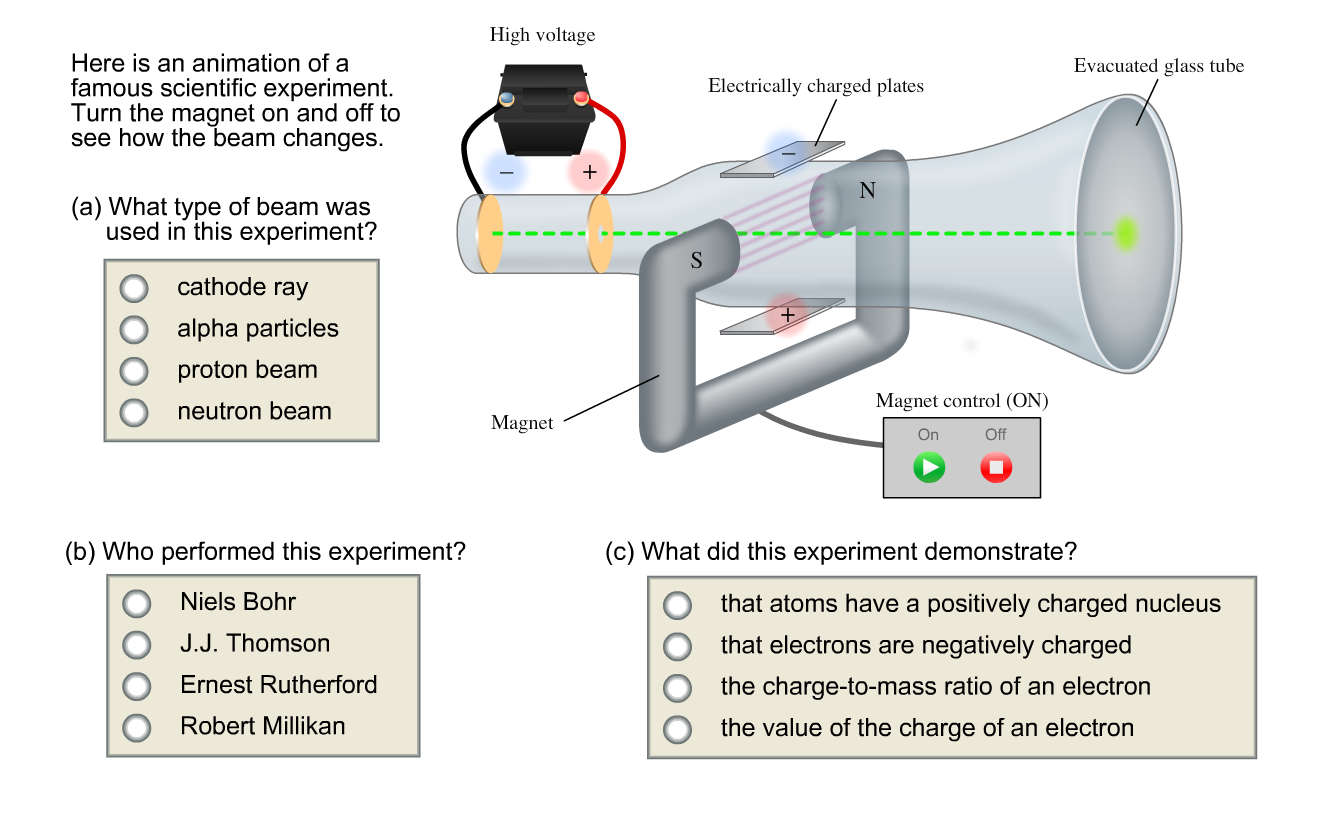
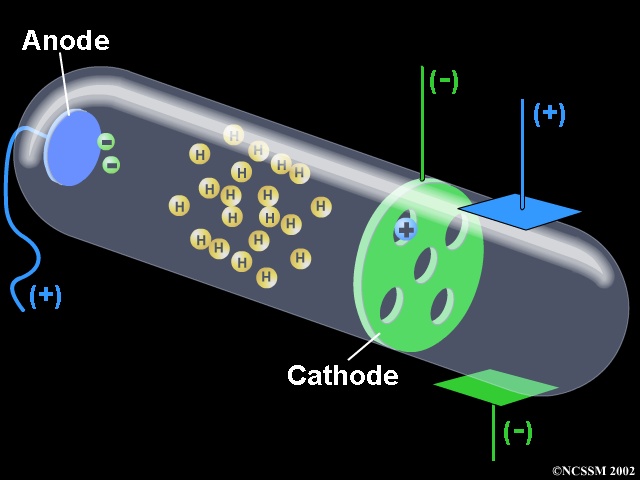
Thomson's cathode ray tube experiment. Canal Ray experiment is the experiment performed by German scientist Eugen Goldsteinin 16 that led to the discovery of the proton. Turn the magnet on and off to see how the beam changes.
Rutherford gold foil experiment. He built his cathode ray tube with a metal cylinder on the other end. It figured out a bunch of details about electrons and specially that the atom is not the smallest particle in matter.
In addition, the experiment could describe characteristic properties, in essence, its affinity to positive charge, and its charge to mass ratio. The diagrams help students understand the significance of each experiment. Cathode Ray Tube Animation.
When a gas is subjected to a high potential (5000 to V) at low pressure, the glass wall of tube glows with fluorescent light. State whether the statement is true or false “A negative high voltage is applied to the filament to emit electrons” and justify your answer?. He did this using a cathode ray tube or CRT.
The rays bent towards the positive pole, indicating that they are negatively charged. Scientists in the early nineteenth century were aware of electricity and the effect of electric potential. Then, on operating the CRT, in the absence of.
CRO is an electronic device that gives graphical representation of alternating quantities under. Crookes also demonstrated that magnetic fields could deflect cathode rays. The activity sheet has diagrams of each of the cathode ray tubes used in the demonstration.
The discovery of electrons in chemistry was substantiated by an electric discharge in cathode-ray tubes. The cathode ray oscilloscope is a vital piece of diagnostic lab equipment for observing and measuring electrical signals at frequencies ranging from dc to GHz Watch full Video. Thomson's cathode ray experiment was a set of three experiments that assisted in discovering electrons.
Cathode ray SmallCircle alpha particles proton beam neutron beam Who performed this experiment?. In an oscilloscope, the CRT produces the electron beam which is accelerated to a high velocity and brings to the focal point on a fluorescent screen. What was the cathode ray tube experiment conclusion?.
A CRO is an electronic device with a CRT as its main component and other associated circuits consisting of a power supply unit, a sawtooth-wave generator, horizon and vertical amplifiers. Thomson took a cathode ray tube and at the place where the electron beam was supposed to strike, he positioned a pair of metal cylinders having slits on them. Regions in a Crookes tube.
The magnitude of the electric current flowing. Thomson's Cathode Ray Tube experiment. Thomson is the first individual to succeed in deflecting the cathode ray with an electrical field.
Sim1 0,0,359,0 360,0,440,0 1 false true standard %CORRECT% The electrons are accelerated to the right and strike the television screen to the right of the anode. Thomson noted that the rays inside the tube were deflected, which was inferred as the presence of a negatively charged particle inside the vacuum tube that made such a phenomenon possible. Electrical Q & A;.
This experiment was performed using a cathode ray tube (Crooke’s tube). E) of an electron. A Crookes tube (also Crookes–Hittorf tube) is an early experimental electrical discharge tube, with partial vacuum, invented by English physicist William Crookes and others around 1869-1875, in which cathode rays, streams of electrons, were discovered.
In 1879, Sir William Crookes demonstrated that cathode rays travel in straight lines by using the tube shown at the right. History of atomic structure. Travelling in straight lines and and producing a shadow from radiated waves indicated a wave nature.
Thomson's cathode ray experiment and Rutherford's gold foil experiment. The electric potential is a driving force that results in the flow of current through a substance due to the difference in concentration of charges at two ends of it. Cathode ray, stream of electrons leaving the negative electrode (cathode) in a discharge tube containing a gas at low pressure, or electrons emitted by a heated filament in certain electron tubes.
You can change the value of the electric field and play it with the magnetic field, of 2 mT, on or off. Cathode Ray Oscilloscope Animation. Cathode Ray Tube Experiment - J.J.
Thomson showed that cathode rays were. Cathode Ray consist of negatively charged particles called electrons. His first experiment was to build a cathode ray tube with a metal cylinder on the end.
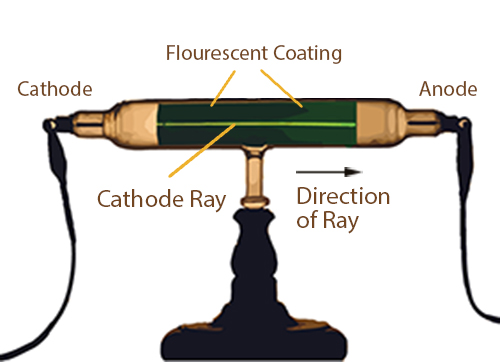
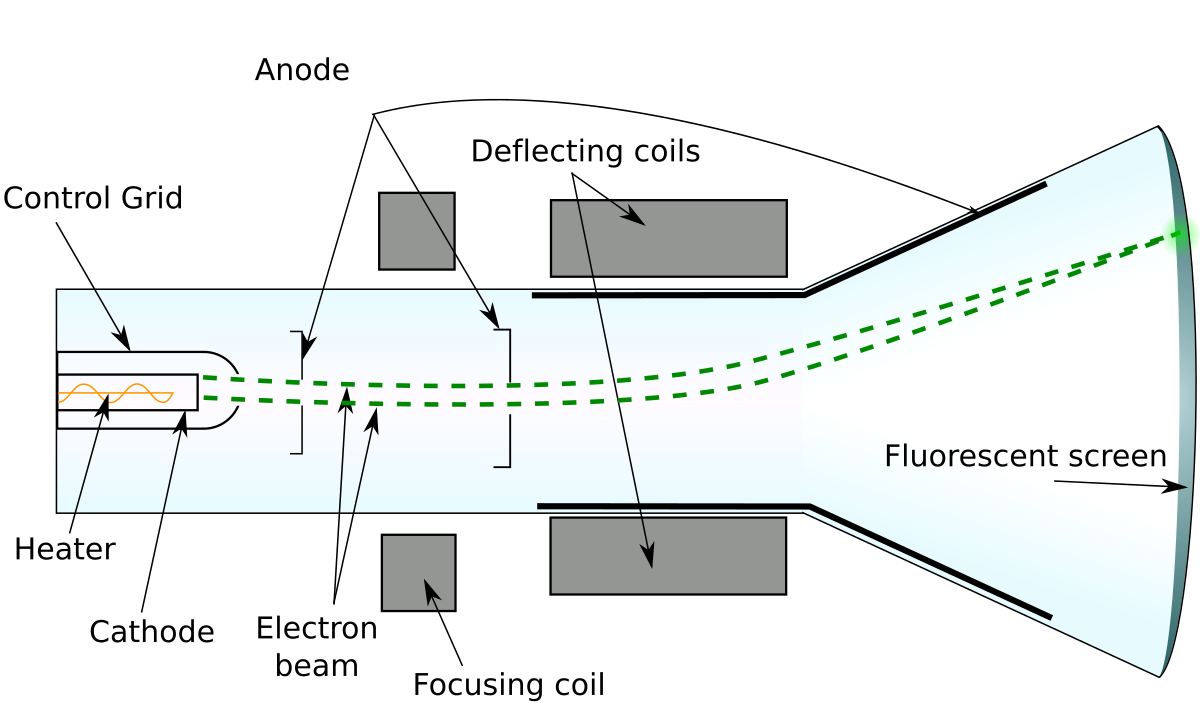
For experiments with cathode ray tube we used an educational model readily available on eBay. Cathode Ray Tube The Cathode Ray Tube (CRT) is a vacuum tube containing an electron gun (a source of electrons) and a fluorescent screen, with internal or ex. It consists of a glass tube connected to two metal electrodes at two ends.
Cathode rays are streams of electrons observed in discharge tubes. Niels Bohr J.J Thomson Ernest Ruthorford Robert Millikan What did this experiment demonstrate?. Apparatus Used A discharge tube was taken in which there were 2 electrodes i.e.
Cathode ray tubes are not an outdated piece of scientific equipment;. The major contribution of this work is the new approach for modelling this experiment, with a great deal of accuracy and precision, using the equations of physical laws to describe the motion of the electrons. This paper describes how J is simulated.
Here is an animation of a famous scientific experiment. Thomson studied cathode ray tubes and came up with the idea that the particles in the cathode beams must be negative because they were repelled by negatively charged items (either the cathode or a negatively charged plate in the cathode ray tube) and attracted by positively charged items (either the anode or the positively charged plate in the cathode ray tube). In the Thomson Cathode Ray Tube Experiment, it was discovered that you can use the deflection of an electron beam in an electric and magnetic field to measure the charge-to-mass ratio (q / m.
Explain what is a cathode ray oscilloscope (CRO)?. Google Classroom Facebook Twitter. Scientists used special vacuum tubes, such as the Crookes tube and the cathode ray tube, to study this phenomenon.
Chapter 8 - Electron Configuration and Chemical Periodicity Millikan Oil Drop Experiment Electron Configs Rutherford's Experiment 1:. It can be used to determine the velocity of the electrons (when they are not deflected by the electric and magnetic fields) and the charge-to-mass ratio. This paper describes the simulation of J.
Thomson was not the only one working on cathode rays, but several other players like Julius Plücker, Johann Wilhelm Hittorf, William Crookes, Philipp Lenard had contributed or were busy studying it. Thomson's Cathode Ray Tube Lab In this lab we will be looking at a model of the Thomson experiment using a Cathode Ray Tube. This is the official Video of Cathode Ray Tube by sir JJ ThomsonA Cathode ray tube is the forerunner of the television tube.
How the deflection of of electrons occur in cathode ray tube?. The history of atomic chemistry. The pair, in turn, was connected to an electrometer, a device for catching and measuring electric charges.
The cathode ray oscilloscope is the instrument which generates the waveform of any electrical quantity. S Bharadwaj Reddy March 4, 15 September 11, 16. Define cathode ray tube with help of a well labeled diagram?.
QUESTION 4 OF 5 A cathode ray tube is the device used in television sets. A CRO is an electronic device with a CRT as its main component and other associated. Cathode Ray Experiment After Dalton's breakthrough of atomic theory, scientists tried to determine the masses of atoms from the fraction of elements in compounds.
The following block diagram shows the general purpose CRO contraction.The CRO recruit the cathode ray tube and acts as a heat of the oscilloscope. Thomson had an inkling that the ‘rays’ emitted from the electron gun were inseparable from the latent charge, and decided to try and prove this by using a magnetic field. Thomson's experiment with cathode rays (position is given in mm, 10 −3 m).
Cathode ray oscilloscope experiment viva questions. 6 The Effect of an Electric Field on. Developed from the earlier Geissler tube, the Crookes tube consists of a partially evacuated glass bulb of various shapes, with two metal.
The animation illustrates the deflection of an electron in external fields which simulates J. Educational atomic physics animation :. Explain what is a cathode ray oscilloscope (CRO)?.
→Anode ray experiment was conducted by E Goldstein. Crookes tube - Maltese cross tube. A Cathode Ray Tube Zumdahl, Zumdahl, DeCoste, World of Chemistry 02, page 58 5 Cathode Ray Experiment.
Anode(+ve) and the cathode (-ve).The tube was filled with an inert gas.A perforated or porous cathode was used. The motion of the electrons can be manipulated and recorded by the user, by assigning different values to the experimental parameters. Using a cathode ray tube, Thomson was able to deflect cathode rays with an electrical field.
Experiments with cathode ray tubes and tried to use the results to determine the nature of cathode rays. How is CRO superior to ordinary measuring instruments?. The tube illustrated in the tutorial contains a negative electrode (Cathode) at one end and a positive electrode (Anode) at the other.A high voltage is transmitted to the cathode ray tube, inducing the cathode to emit electrons – essentially an electrical current.
If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from the cathode. This is the currently selected item. The Maltose cross was placed midway in the cathode ray tube.
4) Although there was some speculation that the cathode rays were negatively charged, it is not shown to be true by experiment until 15, just two years before Thomson announces the electron. Cathode Ray Oscilloscope Block Diagram of CRO. J Thomson, this 50 minute animation looks at every aspect of this discovery.
The activity sheet has embedded questions designed to promote small group discussions. →These rays are also known as canal rays. Since an individual atom is so small, the mass of the atoms of one of the element was determined relative to the mass of the atoms of another element, based on a mass standard.
Thomson’s First Cathode Ray Experiment. The discovery of proton which happened after the discovery of electron further strengthened the structure of the atom.In the experiment, Goldstein applied high voltage across a discharge tube which had a perforated cathode. What type of beam was used in this experiment?.
Discovery of electron took a long journey over hundreds of years from lightning to discharge tubes to cathode rays and finally discovery of electron by J.
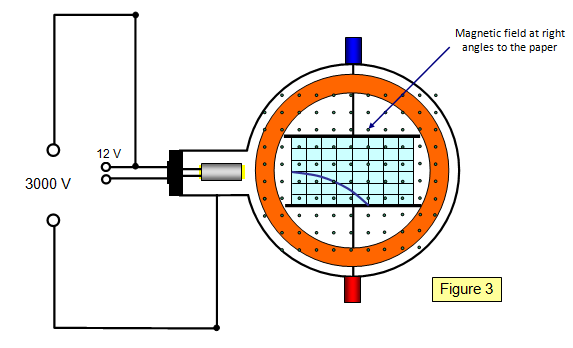
Schoolphysics Welcome
Cathode Ray Oscilloscope And Its Applications On Vimeo

Eeee Storyboard Av 5e161ec8
Gcse Igcse Physics Part1 Cathode Ray Oscilloscope Parts From Cro Cathode Ray Oscilloscope Video On Vimeo
Crookes Tube Stem Resource Finder

Cathode Ray Discharge Tube Experiment Experiments Science Electricity Atomic Structure

Further Understanding Of The Atom Ck 12 Foundation

Learn Electron And It S Discovery In 3 Minutes
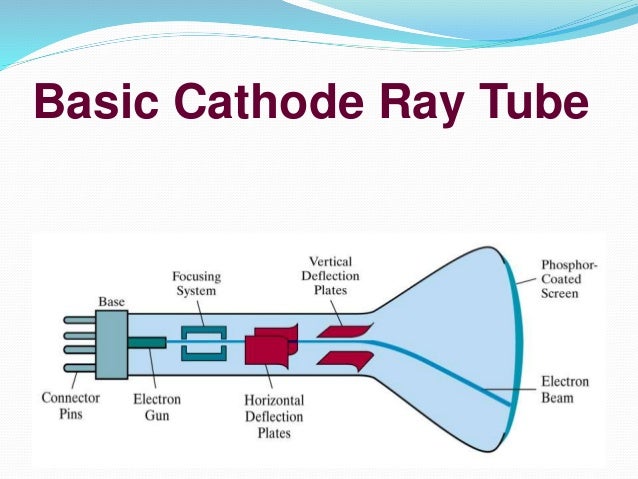
Cathode Ray Tube Technology

Cathode Ray Tube Experiments Physicsopenlab

Discovery Of Cathode Rays And Anode Rays And J J Thomson Atomic Model Youtube
J J Thomson Storyboard By Jegugael1

Who Discovered Electrons The Cathode Ray Experiment Selftution
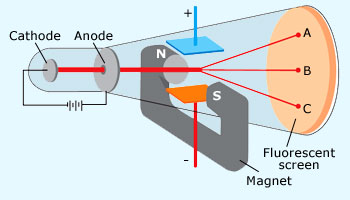
Cathode Ray Experiment

Production Of X Rays

J J Thomson S Cathode Ray Tube Crt Definition Experiment Diagram Video Lesson Transcript Study Com
Q Tbn 3aand9gcsvckcybrl4 2jvdaa4zabnqj 3yxwi9c8ija Usqp Cau
Q Tbn 3aand9gcsqvmicqvowuubf8eiz4vsygpq4autrmbfg0a Usqp Cau
J J Thomson Robert Millikan Atomic Model Project123

Animations List Bauer

Q Tbn 3aand9gcrzjoumg Uigncpmqj9nlf8hwgu Kv87fgsvw Usqp Cau

Cathode Ray Wikipedia

Q Tbn 3aand9gctzoprxub 25tcyls L1i58snkoklxkguaf0q Usqp Cau
Atomic Timeline Project Sutori

Physicslab Famous Discoveries The Franck Hertz Experiment

Cathode Ray Tube Experiment Jj Thomson Cathode Ray Tube Tecnologia

Proton Read Chemistry Ck 12 Foundation

Ernest Rutherford S Gold Foil Experiment Physics Lab Video Lesson Transcript Study Com

Cathode Rays Introduction To Chemistry
Q Tbn 3aand9gcqmmemfuczgla97 Pw5852wdkrhjxz9qosbm3a5n Njggp1f6wk Usqp Cau

Electromagnetic Deflection In A Cathode Ray Tube I Maglab

Electronic Structure Of Atoms Chemistry Library Science Khan Academy

Gcse Igcse Physics Part1 Cathode Ray Tube From Cro Cathode Ray Oscilloscope Video Youtube
J J Thomson

X Rays Storyboard By Valebelieve

Q Tbn 3aand9gcsx9nhq3n8mt Ziat0q B8w17ppv3s4m Glig Usqp Cau

J J Thomson S Cathode Ray Tube Crt Definition Experiment Diagram Video Lesson Transcript Study Com

Q Tbn 3aand9gcrdg9thnraixzh5vsuvomwawza9bjgzkxdlhq Usqp Cau

Interactive Student Tutorial
Q Tbn 3aand9gctsnzmvc G1jsalbowayhttut W Qbmsdlrbbgsbgx1g Ibrx87 Usqp Cau
Ppt A Cathode Ray Tube Powerpoint Presentation Free Download Id
Cathode Ray Electron Discovery Vacuum Experiment Jean Baptiste Perrin Transparent Png

X Ray Production Animation Youtube
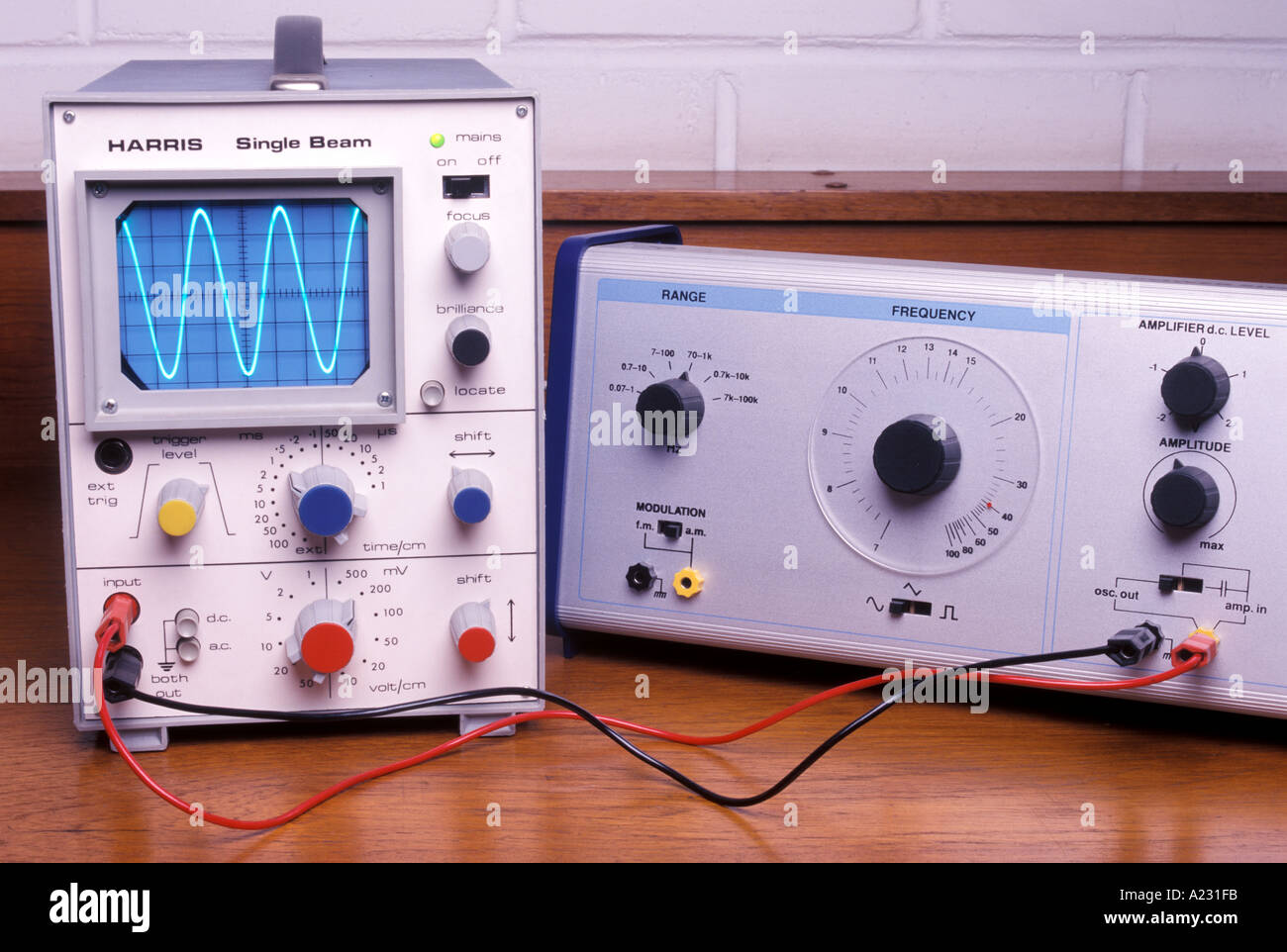
Signal Generator Connected To A Cathode Ray Oscilloscope Or Cro Stock Photo Alamy
Solved Here Is An Animation Of A Famous Scientific Experi Chegg Com

Goalfinder Discovery Of Proton Animated Easy Science Technology Software Online Education Medical K12 Animation E Learning

Atoms And Periodic Properties Ppt Download

J J Thomson S Cathode Ray Tube Crt Definition Experiment Diagram Video Lesson Transcript Study Com

Q Tbn 3aand9gcrlozveqnsexqb9ya54wysplqxzlssxyv9xwq Usqp Cau

Cathode Ray Oscilloscope Cro Electronics Physics Youtube

Excellent Explanation Discovery Of The Electron Cathode Ray Tube Experiment This Really Helped My Kids Understa Apologia Chemistry Basic Physics Experiments
Q Tbn 3aand9gcssph7sv9kk8r75onswscfxz 6f1otb2syn G Usqp Cau
Q Tbn 3aand9gcqg Q366kmi14xzv1z0yrnf Hgnn6 X0nu23q Usqp Cau

What Are Cathode Ray Experiment And Anode Ray Experiment Chemistry Topperlearning Com Oxijwfuu

Chemistry 2 Storyboard By 6d7057b5
Q Tbn 3aand9gcthtcu5v0ohqe2 Fq30y366bkhonsdhch8yofd Hqov6vnpcdvg Usqp Cau

Q Tbn 3aand9gcrmzassioyu7r6emebwdpo8oi8zeokv8 Octa Usqp Cau
Cathode Ray Tube Youtube

Cathode Ray Tube Animated Explanation Youtube
Goalfinder Discovery Of Proton Animated Easy Science Technology Software Online Education Medical K12 Animation E Learning

Cathode Ray Wikipedia

Learn Electron And It S Discovery In 3 Minutes
Go Aws 34vpqhp

Cathode Ray Discharge Tube Construction Youtube
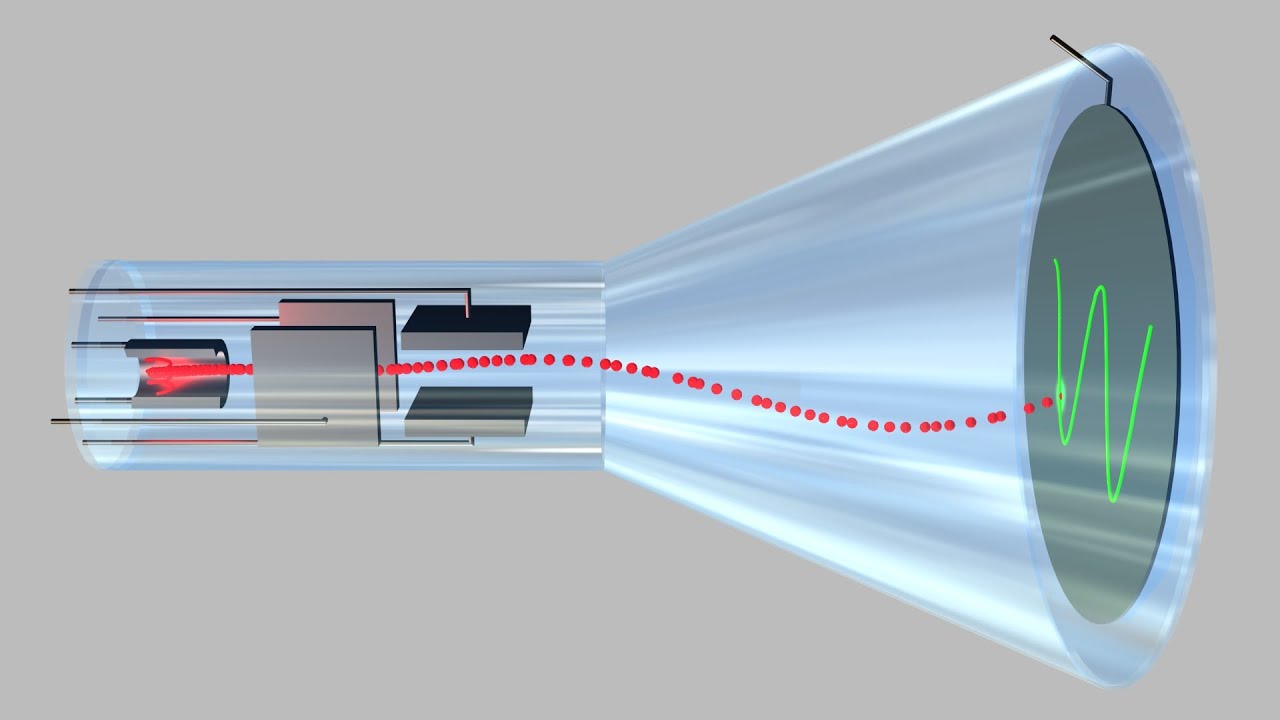
541 Cathode Ray Tube Of Oscilloscope Youtube
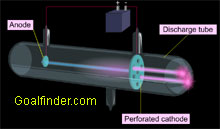
Goalfinder Discovery Of Proton Animated Easy Science Technology Software Online Education Medical K12 Animation E Learning

3d Animation Cathode Ray Tube 19 Youtube

Charge To Mass Ratio Electron Class 11 Chemistry Youtube

Discharge Tube Experiment And Properties Of Cathode Rays

Media Portfolio

Animations Produced And Used In The Research Download Table
Models Of The Atom Animation Annenberg Learner
Cathode Ray Oscilloscope

Atomic Theory I Chemistry Visionlearning

Cathode Ray Wikipedia
Cathode Ray Tube Definition Charatersitics Diagram Youtube
Tiger Ncssm Distance Education And Extended Programs
Tiger Ncssm Distance Education And Extended Programs
File Cathode Ray Tube Diagram En Svg Wikimedia Commons

Discovery Of Electron Cathode Rays Discharge Tube Experiment Full Chemistry Animation Facts Youtube

Atomic Discovery Early Models Of The Atom 400 B C Democritus Proposed The Existence Of Fundamental Particles Of Matter That Were Indivisible And Indestructible Ppt Download
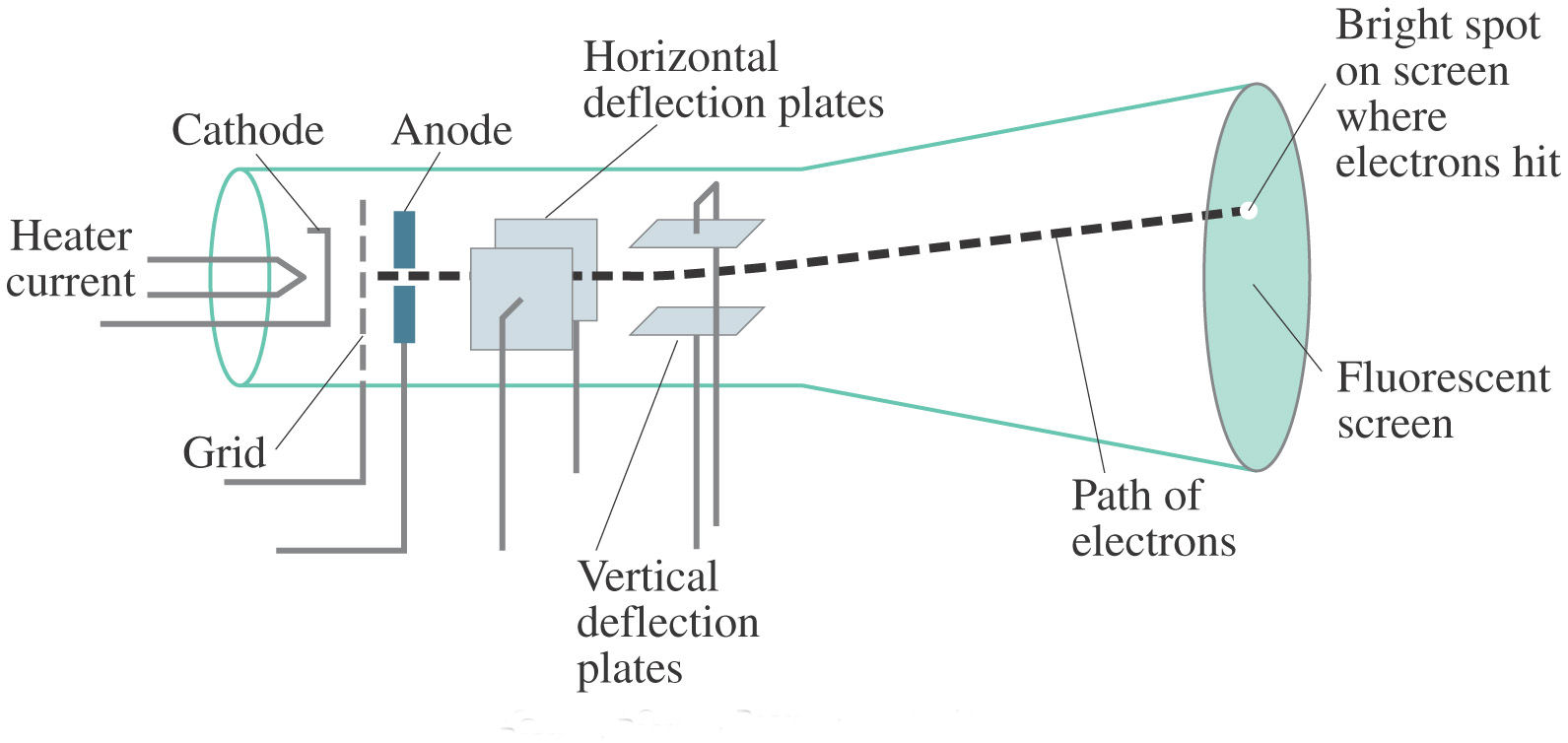
Cathode Ray Tube Crt Science Facts
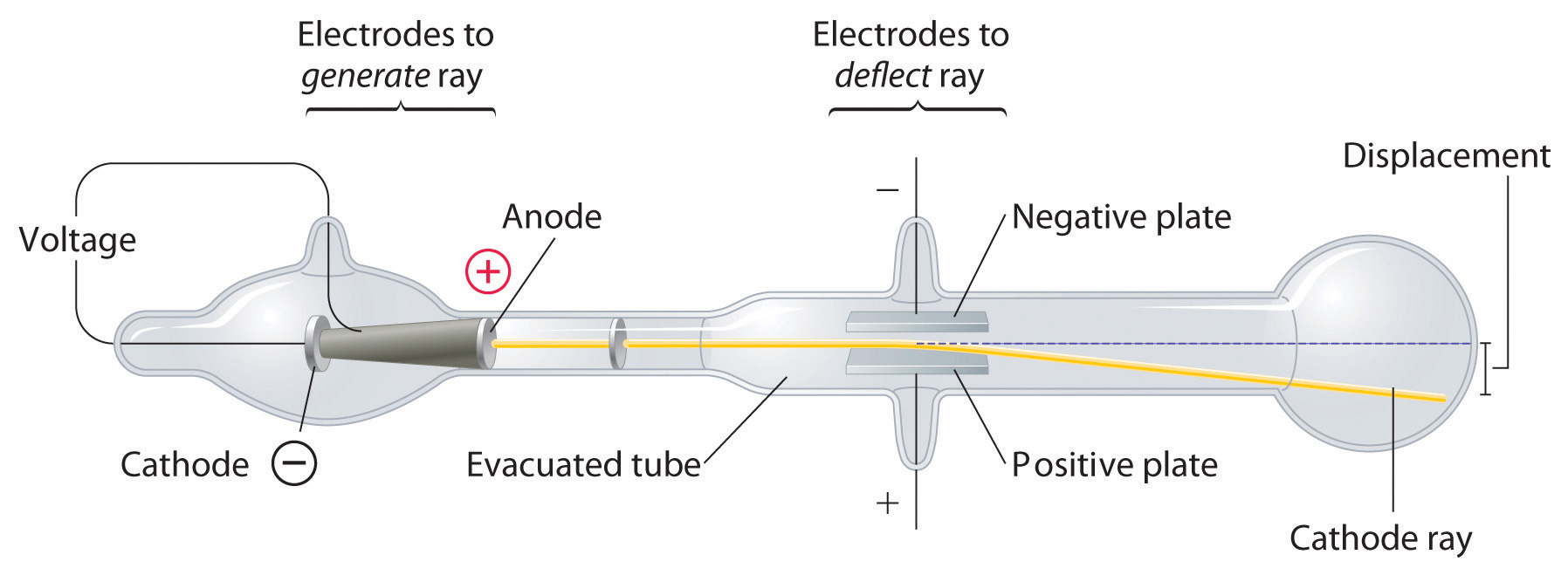
The Atom

Modern Theories Of The Atom Ppt Download

Thomson S Cathode Ray Tube Experiments Youtube

Crookes Tube Wikipedia

551 Millikan Experiment On A Single Electron Charge Measurement Oil Drop Experiment Experiments Electrons
Ieeexplore Ieee Org Iel7 Pdf

Learn Electron And It S Discovery In 3 Minutes

Cathode Ray Experiment Summary Explanation Video Lesson Transcript Study Com

Media Portfolio

2 4 Early Experiments To Charachterize The Atom Apchemwins

Atomic Structure
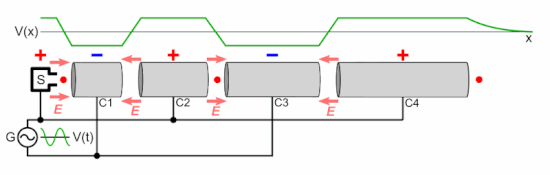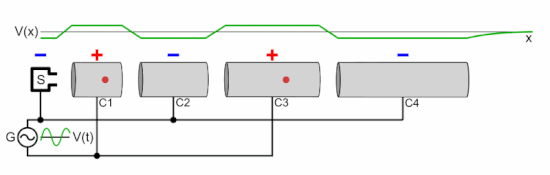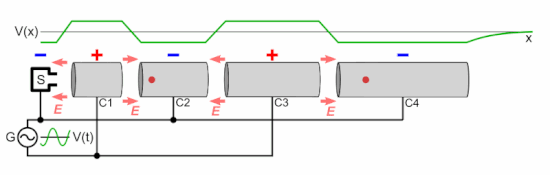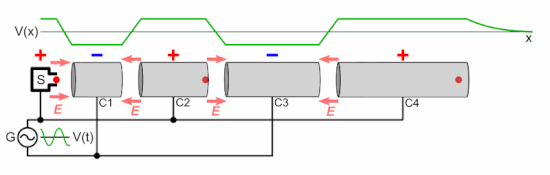
Q Tbn 3aand9gcsz8gzafh8bsl5zauw4wlvolydyu8snbidxrg Usqp Cau

Cathode Ray How Does It Work Animated With 3d Youtube

Q Tbn 3aand9gcs4tjagl1f0ppqzombrcfffvshoxhta Feqeq Usqp Cau

Cathode Ray Tube Youtube
Q Tbn 3aand9gcslplcpp4hpif3f0owc7mfxuup1bc O1pqltzqtsqnp7yzkrglq Usqp Cau



